Instruments to control water quality
In food, aquaculture, fish farming and agriculture it is essential to control the properties of water or certain solutions

In a liquid medium there are a set of variables that act as indicators of the quality or state of that medium. When these variables or values are altered, it is usually synonymous with microbiological activity or some external alteration.
In agriculture, vegetables have a tolerance margin to certain substances or chemical components, if this margin is exceeded, the medium becomes toxic. The same happens in aquariums or pools that contain fish or other animals, or obviously also in substances for human consumption.
Some of these variables or indicators are:
PH: The acidity or alkalinity of the medium betrays us, as a consequence of the concentration of hydrogen ions. The PH range is from 0 to 14, with PH 7 being a neutral value. Both very basic and very acidic values imply toxicity for living beings.
Conductivity: It is used to determine the concentration of salts or cations in an aqueous medium. At higher conductivity values, higher concentration and also higher salinity.
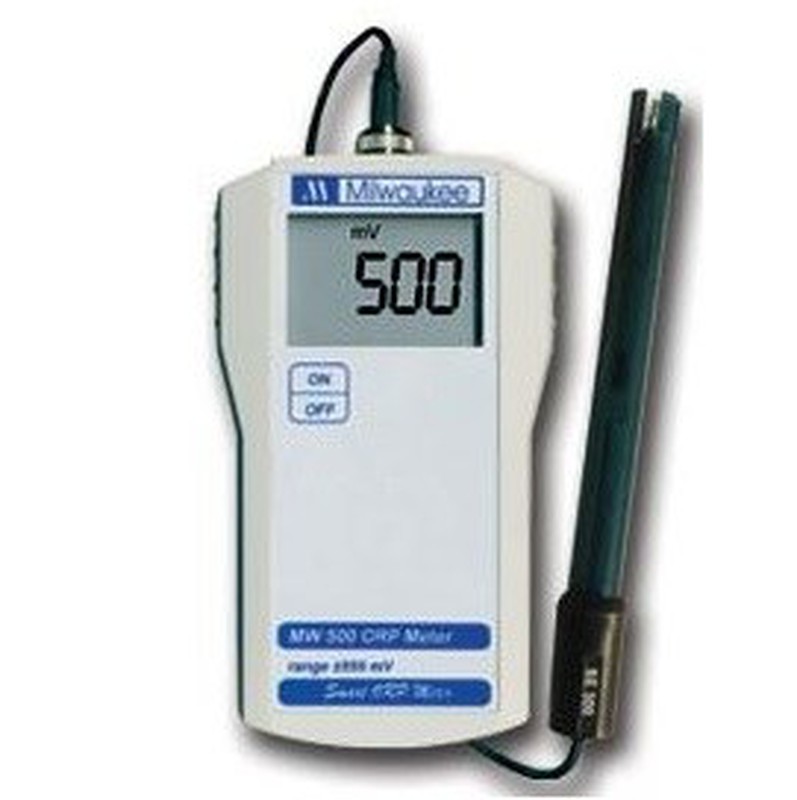
Oxido-reduction: The oxide-reduction processes are the natural system of exchange of electrons between two bodies or media, the one that oxidizes loses them and the one that reduces gains them. This process can be synonymous with a change in the concentration of salts or solutes or biological activity.
Dissolved oxygen: The concentration of oxygen in water is essential for aquariums, ponds and tanks where there are living beings, determines their survival. In reservoir fresh water or even wastewater, the oxygen concentration determines the quality of the water and indicates the present microbiological activity.
Chlorine: Chlorine is an element that is used to disinfect swimming pools, but it can also be a symptom of toxicity of water for human consumption.
We have a wide variety of instruments for the control of all these variables, they can be portable or laboratory devices, but all of them will accurately determine the value that interests us.
Portable devices are very useful for field work or for inspections in the same control environment. With laboratory devices, the control is much more exhaustive and even allows the visualization and storage of data directly on a computer.
The instruments are supplied with a predetermined probe and maintenance and calibration solutions for the probe electrodes.
The control of these variables is carried out on liquid medium or on very soft solids such as creams.
In food: Variables such as PH or conductivity can help us determine whether certain liquid foods are suitable for consumption or not, or whether they have optimal properties at a specific point in production, for example in cheeses.
In aquaculture and aquariums: We need to have a comprehensive control of the salinity, pH and amount of oxygen dissolved in water. Fish, algae, etc. need specific values for their survival, if they are exceeded the environment becomes toxic and therefore would compromise the life of these animals.
In swimming pools: Pool water must meet the necessary conditions for bathing, here values such as conductivity, salinity, pH or chlorine level are essential to keep the pool disinfected and clean.
In water treatment centers: To control the purification process of water for human consumption, the control of PH, salinity, conductivity, chlorine or oxygen values are essential. We need to avoid the presence of microbial agents.
In the environment: To control the quality of the water in aquifers, ponds, lakes, marshes, etc. Know if the water presents symptoms of eutrophication and, for example, poor or very poor oxygen values that could compromise life inside.





Opinions of our clients
Receive our news